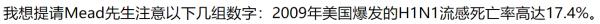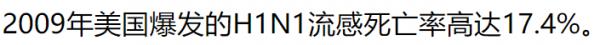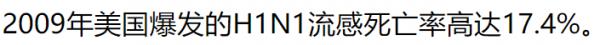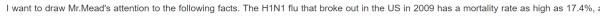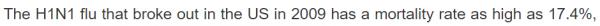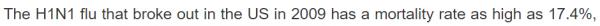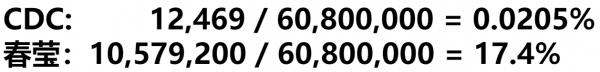| 春莹津津乐道美国09年流感死亡率17.4%! |
| 送交者: Pascal 2020年02月12日02:45:44 于 [五 味 斋] 发送悄悄话 |
|
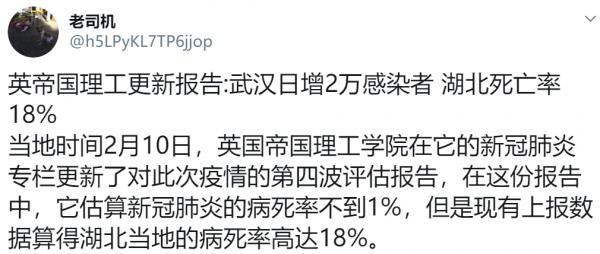
CDC: 12,469 / 60,800,000 = 0.0205% 春莹:10,579,200 / 60,800,000 = 17.4%
*******************************
视频链接: https://twitter.com/chuaijiji/status/1227392733624451072
***************************
Isolation of a new bat virus in a study published in the Journal of Virology on Dec. 30, 2015, titled “Isolation and Characterization of a Novel Bat Coronavirus Closely Related to the Direct Progenitor of Severe Acute Respiratory Syndrome Coronavirus,” found that the virus, named SL-CoV-WIV1, was almost identical to Rs3367 with 99.9% genome sequence identity. The researchers identified that WIV1 can use human ACE2 as an entry receptor and has the potential to infect human cells in this study. Subsequently, the same research group isolated another bat virus that can use ACE2 and infect human cell lines in the lab in 2015. In addition, Dr. Shi’s group conducted another study in 2018 to address the question of whether some bat viruses can infect humans via using human ACE2, without the need of an intermediate host. But to the date of their study, “no direct transmission of SARS-Like CoVs from bats to people has been reported”. 2015年12月30日发表在《病毒学杂志》上的一项研究中,名为「与严重急性呼吸系统综合症冠状病毒直接源细胞密切相关的新型蝙蝠冠状病毒的分离和鉴定」的研究发现了一种新的病毒,名为SL-CoV-WIV1,其基因与Rs3367几乎相同,具有99.9%的基因组序列相似性。研究人员确定,WIV1可以使用人类ACE2作为进入受体,并有可能感染人类细胞。随后,同一研究小组于2015年在实验室中分离出了另一种可以使用ACE2并感染人类细胞系的蝙蝠病毒。 此外,石博士的小组于2018年进行了另一项研究,以解决一些蝙蝠病毒是否可以通过使用人ACE2感染人类而无需中间宿主的问题。但是到迄今他们的研究,「尚未有类似SARS的冠状病毒可以从蝙蝠直接传给人类的报导」。 Zhengli Shi’s group at the Institute of Virology at Wuhan was successful in isolating two infectious clones of bat SARS-Like CoV: SL-CoV-WIV1 and WIV16 from bats. In their further studies, they found out that these SL-CoV Spike protein (S protein) “[were] unable to use any of the three ACE2 molecules as its receptor; Second, the SL-CoV failed to enter cells expressing the bat ACE2; Third, the chimeric S covering the previously defined receptor-binding domain gained its ability to enter cells via human ACE2, albeit with different efficiencies for different constructs; Fourth, a minimal insert region ( Amino acids 310 to 518 ) was found to be sufficient to convert the SL-CoV S from non-ACE2 binding to human ACE2 binding.” Therefore, Shi’s group found in a study published in the Journal of Virology in February 2008 that the natural bat coronavirus cannot use the human ACE2 receptor to infect humans. However, when inserted with some amino acids from position 310 to 518 for the bat CoV S protein sequence, the chimeric bat CoV can use the human ACE2 receptor. Meanwhile, another research group led by Dr. Li published their finding in 2013 that 5 amino acid sites on CoV spike proteins are crucial in making the binding to human ACE2 on SARS virus (those positions are Y442, L472, N479, D480, T487). These 5 sites just lie in the region that the Shi group noted to be important above. Later, Li and Shi jointly conducted a gain-of-function study published in the Journal of Virology in September 2015 on the MERS virus and a bat virus (strain HKU4) in 2015. Since MERS virus can enter human cells but HKU4 can not, they introduced 2 single mutations in the HKU4 spike protein and found that the new mutant S protein can enable HKU4 to enter human cells. If they mutated 2 sites in MERS spike, the resulting MERS pseudovirus (experimental virus) cannot enter human cells anymore. 武汉市病毒研究所的石正丽研究小组成功地从蝙蝠中分离出两个蝙蝠SARS样冠状病毒的传染性克隆:SL-CoV-WIV1和WIV16。在进一步的研究中,他们发现这些SL-CoV 突触蛋白(S蛋白)「无法使用三种ACE2分子中的任何一种作为其受体。其次,SL-CoV无法进入表达蝙蝠ACE2分子的细胞中。第三,覆盖先前定义的受体结合域的嵌合体S蛋白通过人类ACE2分子获得了进入细胞的能力,尽管对不同构建体的效率不同。第四,发现最小的插入区(氨基酸310至518)足以将SL-CoV S蛋白从非ACE2分子结合转化与人类ACE2分子结合。」 因此,石的小组在2008年2月发表在《病毒学杂志》上的一项研究中发现,天然蝙蝠冠状病毒无法利用人类ACE2受体来感染人类。但是,当在蝙蝠CoV S蛋白序列310到518的位置插入一些氨基酸时,嵌合体蝙蝠CoV可以使用人类ACE2受体。 同时,由李博士领导的另一个研究小组于2013年发表了他们的发现,冠状病毒突触蛋白上的5个氨基酸位点对SARS病毒与人类ACE2的结合至关重要(位置分别为Y442,L472 ,N479,D480,T487) 。这五个点就在石小组在上面指出的重要区域内。 后来,李和石于2015年9月在《病毒学杂志》上联合发表了项「功能获得」的研究。该研究于2015年针对MERS病毒和一种蝙蝠病毒(HKU4株)进行了研究。他们在HKU4突触蛋白中引入了2个单突变,发现新的突变S蛋白可以使HKU4进入人体细胞。如果他们在MERS S蛋白突变2个位点,则产生的MERS伪病毒(实验病毒)将无法再进入人类细胞。 To their surprise, the chimeric virus (SHC014-MA15) can use SHC014 spike to bind to human ACE2 receptor and enter human cells. SHC014-MA15 can also cause disease in mice and cause death as well. Existing vaccines to SARS cannot protect animals from SHC014-MA15 infection. Therefore, these chimeric virus studies can lead to the generation of more pathogenic, more deadly CoV strains in mammalian models. Due to the U.S. government-mandated pause on the gain-of-function (GOF) studies, this international research did not proceed further at that time. However, there is no evidence that Shi’s group in China stopped any further study on the track of introducing GOF mutations on the CoV. And it is clear that Shi’s group already mastered the reverse-engineering technology that is sufficient to introduce mutation in current SARS-CoV or SARS-Like CoV to create mutant infectious coronavirus. 令他们惊讶的是,嵌合病毒(SHC014-MA15)可以使用SHC014突触结合人类ACE2受体并进入人的细胞。 SHC014-MA15也可引起小鼠疾病并引起死亡。现有的SARS疫苗不能保护动物免受SHC014-MA15感染。因此,这些嵌合病毒研究可导致在哺乳动物模型中产生更致病,且更致命的冠状病毒株。 由于美国政府强制暂停「功能获得」(GOF)研究,因此该国际研究当时并未进一步进行。但是,没有证据显示石博士在中国的小组停止了在CoV中引入GOF突变的进一步研究。很明显,石的研究小组已经掌握了逆向工程技术,该技术足以在当前的SARS冠状病毒或SARS 样冠状病毒中引入突变以产生突变型的传染性冠状病毒。
t has been two months since the outbreak of the coronavirus in Wuhan and its spread has shown no signs of slowing down in China. More than 35 Chinese cities have been put on lockdown by Chinese authorities in an attempt to isolate confirmed and suspected cases. The lives of millions of people are in danger as the virus shows signs of spreading further in China as well as internationally. There are significant gaps in the official investigations into the origins of the novel Coronavirus. In order to contain the virus, one first needs to understand how a virus that allegedly originated in animals found its way to humans. For this to happen, the Chinese authorities need to release their animal testing data and samples. Testing results from animal samples collected at epicenters would give important insights into what animals might serve as intermediate hosts for the new coronavirus. This is critical to the containment of the epidemic. For example, if rats are the intermediate hosts for this virus, it would be futile to shut down the cities to restrict people’s movements while infected rats are still moving freely. Results from animal samples could also guide policy decisions that would reduce the risk of another outbreak. An Animal Origin of the VirusScientific studies based on phylogenetic analysis have researched the sequence of the novel coronavirus, compared it to other coronavirus sequences, and found it likely originated in bats. Researchers from the Wuhan Institute of Virology found the genome in the virus found in patients was 96 percent identical to that of an existing bat coronavirus, according to a study published in the journal Nature. But there have been other theories as well. One Chinese study suggested, for example, that snakes were the source of transmission to humans. However, many scientists believe that reptiles are a less likely source and that mammals like rats and pigs, and some birds, have been the primary reservoir for coronaviruses. With this in mind, phylogenetic studies of viral genome sequences need to be supported by animal studies to confirm the origin of the infection, as well as to determine whether there is an intermediate host. It is not an easy task for a virus to establish zoonotic transmission, and coronaviruses rarely leap from animal to human infection with high transmissibility. There is even less chance to see a coronavirus leap directly from bats to humans. To infect new hosts, mutations need to occur with the viral surface proteins and/or envelope and structural genes, so that the mutated viruses can bind and enter the cells of new species, and efficiently complete the replication cycles in the new hosts. Some scientists have argued that coronaviruses can jump directly to humans, without mutating or passing through an intermediate species. However, an intermediate host was clearly needed to establish zoonotic transmission to humans in the previous outbreaks of coronaviruses. Many studies suggested that the bat coronavirus jumped from its natural host bats to civets and then to humans during the 2003 SARS outbreak, and it jumped from bats to camels and then to humans for the MERS outbreak. So, civets and camels would serve as intermediate hosts for zoonotic transmission. Because bats were not sold at the Huanan market in Wuhan—the epicenter of the infection—at the time of the outbreak, this suggests the existence of another intermediate animal host that may have transferred the virus to humans. What is the most puzzling is that there have been no reports on the testing of animal samples collected in any epicenters in Wuhan, especially at the Huanan seafood market, to identify what animals might be the host or intermediate hosts of this novel Wuhan coronavirus. Chinese scientists published a report in Lancet recently which stated that “the majority of the earliest cases included reported exposure to the Huanan Seafood Wholesale Market” and that patients could have been infected through zoonotic or environmental exposures. Another report on Lancet by Chinese CDC scientists claimed that “on the basis of current data, it seems likely that the 2019-nCoV causing the Wuhan outbreak might also be initially hosted by bats, and might have been transmitted to humans via currently unknown wild animal(s) sold at the Huanan seafood market.” However, so far, no information was released about the amount, and species, of wild animals present at the Huanan seafood market upon closure; nor about how the animals were managed or disposed of when the market was closed on Jan. 1, 2020. And no information was released about how many animal samples were tested for SARS-CoV or Wuhan Coronavirus via viral nucleic acid testing methods. Official Chinese state news agency Xinhua reported on Jan. 26 that 33 samples out of 585 environmental samples collected at the Huanan Seafood market were positive for nucleic acids from new Coronavirus, suggesting the virus originated from wild animals or stocks sold there. However, these samples were from the environment—not from animals. It would be an ultimate failure of the Wuhan public health commission and Chinese CDC if no animal samples were collected and tested prior to, or at the time of, the shutting-down of the Huanan seafood market, where many animals were sold at the time of the outbreak. It would be similar to conducting an investigation on a food-borne disease outbreak without taking restaurant food samples related to the outbreak, and instead taking dining table surface swabs to test. Background of the Huanan Seafood Market ClosureThe 2019-nCoV has caused rapid infection in China and spread to other countries outside China, which has led to a global health crisis. The Huanan seafood market is known to be a major outlet for the collection and distribution of live and dead wild animals. These included live wolves, hedgehogs, deer, birds, snakes, goats, hares, and boars that were sold and available in the east section of the seafood market. A Wuhan medical and health committee identified multiple pneumonia cases associated with Huanan seafood market, which were announced on Dec. 31, 2019. The seafood market was closed by the Wuhan government on Jan. 1. Chinese medical reporters visited the market on Dec. 31, 2019, the evening before its closure on Jan.1 where they observed poor hygiene, and wild animal bodies and organs disposed of in an unorganized manner. This suggested that a relatively large quantity of wild animals were still present at the market upon the forced closure. No Information on Wild Animals at the Seafood Market Was DisclosedYet, no information was released about the amount, and species, of animals present upon closure, how many animals were tested for Coronavirus, and how the animals were managed or disposed of upon the closure of the market on Jan. 1. A Chinese media outlet, Yicai, inquired about the outcome of the wild animals sold at the market and confirmed that there was no disclosure from the Wuhan government. Dr. Guan Yi, the current director (China affairs) of the State Key Laboratory for Emerging Infectious Diseases at the University of Hong Kong, visited Wuhan on Jan. 21 with the goal of identifying the animal source. He mentioned in a media interview that locals refused to cooperate with him. He pointed out that with the market now closed, it would be difficult to investigate the origin of the virus. He said the “Huanan seafood market was cleaned after the closure, ‘the crime scene’ was gone, and how can you solve a case without evidence?” Gao Fu, director of the Chinese Center for Disease Control and Prevention stated, “it is clear that the source of infection was from wild animals, but we don’t know which species due to closure of the seafood market.” The Huge Risks of Not Identifying the Original or Intermediate Animal HostsThe U.S. Center for Disease Control (CDC) stated that “much is unknown about how 2019-nCoV, a new coronavirus, spreads.” So far the understanding is that the major pathway of 2019-nCoV infection is respiratory droplet transmission and contact from humans to humans. Guan Yi and Kwok-yung Yuen of the University of Hong Kong (HKU) et. al. identified severe acute respiratory syndrome coronavirus (SARS-CoV) from caged palm civets from live animal markets in China in 2003. Their studies lead to the subsequent ban on selling civets and the closing of all wild animal markets in Guangdong and helped to confine the SARS epidemic. Typically, if an animal is identified as host or source of spread of disease, authorities and the CDC would initiate prevention and control measures such as an awareness campaign, proper quarantine of sick animals and disposal of carcasses as well as monitoring the potential route for how the disease spread zoonotically. Rodents are known to infest seafood markets. For example, tens of thousands of rodents are expected to be unleashed in Japan as one big fish market is closed. Huanan seafood market is also infested by rodents. If rodents were elucidated as being a potential host for coronavirus, the risk of rats infesting beyond the current quarantine zone still persists. Given the fact that coronavirus was detected from feces from patients from Shenzhen and that bat SARS-like virus strains were isolated from bat feces, the possible fecal-oral route of 2019-nCoV transmission in addition to respiratory droplet transmission would lead to a reasonable warning for people to avoid contact with animals like rats. Thus, if rodents are indeed a source or host of the 2019-NCoV infection, then, rodent contamination of food or water is a potential way for the disease to spread, which needs to be brought to the awareness of the international community. Similarly, if birds or other species were the hosts of 2019-nCoV in the seafood market, the information pertaining to the species, amount, virus type, biological reactions, and potential routes of spreading of the virus also need to be identified or reported to the world so that appropriate prevention measures could be taken. It would be serious incompetence and malfeasance if Chinese authorities did not attempt to collect nasal, fecal, and blood samples from animals and birds sold at the seafood market. Testing animal samples would reveal very important information regarding the zoonotic transmission routes, the trends of viral mutations in this outbreak, and the loopholes in the current countermeasures. Were There Other Epicenters Besides the Huanan Seafood Market?The Chinese CDC did release data from environmental samples from the seafood market and suggested that “it is originated from wild animals with species uncertain.”
The Possibility 2019-nCoV Originated From Bat SARS-Like Virus (Bat-SL-CoV)One recent Lancet report on Jan. 29, 2020, titled “Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding,” stated that “A Blast search of the complete genomes of 2019-nCoV revealed that the most closely related viruses available on GenBank were bat-SL-CoV-ZC45 (sequence identity 87.99%; query coverage 99%) and another SARS-like betacoronavirus of bat origin, bat-SL-CoV-ZXC21 (accession number MG772934;23 87.23%;” “Notably, the 2019-nCoV strains were less genetically similar to SARS-CoV (about 79%) and MERS-CoV (about 50%).” This message might be interpreted as 2019-nCoV being biologically closer related to SARS-like betacoronavirus of Bat origin and bats may be the original host of this virus. However, the authors did not claim that the only host to 2019-nCoV is a bat.
They mentioned that most bats in Wuhan are hibernating and no bats are sold at the Huanan seafood market. Thus, the chance of physical contact from bats to spread the virus to humans or animals at Wuhan is highly unlikely. Studies From Wuhan Institute of Virology on Bat SARS-Like CoV.Zheng-Li Shi and several other researchers at the Wuhan Institute of Virology published an article in Nature in 2013 titled “Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor.” In that study, their team harvested from anal swabs or fecal samples from bats and found 2 strains of sequences from Bat SARS-Like CoV that termed as RsSHC014 and Rs3367. They process 95% nucleotide sequence identity with human SARS-CoV Tor2 strain. Isolation of a new bat virus in a study published in the Journal of Virology on Dec. 30, 2015, titled “Isolation and Characterization of a Novel Bat Coronavirus Closely Related to the Direct Progenitor of Severe Acute Respiratory Syndrome Coronavirus,” found that the virus, named SL-CoV-WIV1, was almost identical to Rs3367 with 99.9% genome sequence identity. The researchers identified that WIV1 can use human ACE2 as an entry receptor and has the potential to infect human cells in this study. Subsequently, the same research group isolated another bat virus that can use ACE2 and infect human cell lines in the lab in 2015. In addition, Dr. Shi’s group conducted another study in 2018 to address the question of whether some bat viruses can infect humans via using human ACE2, without the need of an intermediate host. But to the date of their study, “no direct transmission of SARS-Like CoVs from bats to people has been reported”. They collected serum from 218 residents who live close to bat caves with bats carrying the viruses. Those caves were the places where the Shi group collected the virus samples. Then ELISA assays were conducted to detect antibodies to bat SARS-CoV, since antibody existence would suggest a prior exposure to the bat coronavirus. They found that only 6 out of 218 (2.7 %) subjects showed seropositivity, which suggested likely infections to bat SARS-CoVs or related viruses. No clinical symptoms have been manifested in the 6 positive persons in the past 12 months. As a control, they collected 240 samples from random blood donors in Wuhan, 1000 km away from Yunnan, none of the Wuhan blood samples showed any positivity to bat SARS-like CoV. This data suggests that the chance of bat virus infecting humans is very low, <2.9% if possible, and with no obvious symptoms in human beings that live very close to the bat caves. No infection from a bat to a human has been reported in Wuhan as of 2018.
Track Record of Wuhan Institute of Virology on Engineering ‘Gain-of-Function’ Bat SARS-Like CoV.Therefore, Shi’s group found in a study published in the Journal of Virology in February 2008 that the natural bat coronavirus cannot use the human ACE2 receptor to infect humans. However, when inserted with some amino acids from position 310 to 518 for the bat CoV S protein sequence, the chimeric bat CoV can use the human ACE2 receptor. Meanwhile, another research group led by Dr. Li published their finding in 2013 that 5 amino acid sites on CoV spike proteins are crucial in making the binding to human ACE2 on SARS virus (those positions are Y442, L472, N479, D480, T487). These 5 sites just lie in the region that the Shi group noted to be important above. Later, Li and Shi jointly conducted a gain-of-function study published in the Journal of Virology in September 2015 on the MERS virus and a bat virus (strain HKU4) in 2015. Since MERS virus can enter human cells but HKU4 can not, they introduced 2 single mutations in the HKU4 spike protein and found that the new mutant S protein can enable HKU4 to enter human cells. If they mutated 2 sites in MERS spike, the resulting MERS pseudovirus (experimental virus) cannot enter human cells anymore. Furthermore, Shi’s group joined an international group to generate a chimeric virus with the bat virus SHC014 they harvested in Yunnan. Since they know SHC014 is unlikely to bind to human ACE2, they “synthesized the SHC014 spike in the context of the replication competent, mouse-adapted SARS-CoV backbone”. So, that is a lab-engineered virus with SARS-CoV Mouse adapted backbone (MA15) but with SHC014 spike. To their surprise, the chimeric virus (SHC014-MA15) can use SHC014 spike to bind to human ACE2 receptor and enter human cells. SHC014-MA15 can also cause disease in mice and cause death as well. Existing vaccines to SARS cannot protect animals from SHC014-MA15 infection. Therefore, these chimeric virus studies can lead to the generation of more pathogenic, more deadly CoV strains in mammalian models. Due to the U.S. government-mandated pause on the gain-of-function (GOF) studies, this international research did not proceed further at that time. However, there is no evidence that Shi’s group in China stopped any further study on the track of introducing GOF mutations on the CoV. And it is clear that Shi’s group already mastered the reverse-engineering technology that is sufficient to introduce mutation in current SARS-CoV or SARS-Like CoV to create mutant infectious coronavirus.
Interestingly, Shi’s group published on bioRxiv on Jan. 23, 2020 that a new bat coronavirus that they detected in Yunnan, named BatCov RaTG13, shares 96.2 percent overall genome sequence identity with 2019-nCoV. However, this virus was never mentioned or published in their research before. In the sequence information provided by them in the supplemental material and method section, 3 sequences are shared between the 2019-nCoV they collected and the RATG13 virus but not in any of the other SARS or Bat SARS-Like CoV families in the paper listed. The 3 sequences are located close to N terminus of the spike protein, they are GTNGTKR, NNKSWM, RSYLTPGD. Possibilities of an Animal Host of 2019-nCoV at Huanan Seafood MarketOne recent Lancet paper titled “Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding,” reported that “as a typical RNA virus, the average evolutionary rate for coronaviruses is roughly 10⁴ nucleotide substitutions per site per year, with mutations arising during every replication cycle. It is, therefore, striking that the sequences of 2019-nCoV from different patients described here were almost identical, with greater than 99.9% sequence identity. This finding suggests that 2019-nCoV originated from one source within a very short period and was detected relatively rapidly.” With mutations in every cycle, it is highly unlikely for different bats to host viruses with the same sequence. If bats alone are not enough for virus transmission, another animal is needed as the intermediate host, and the chance of the virus being identical is even slimmer. Since the seafood market is not the only source for the outbreak, it is reasonable to postulate that if another animal is the intermediate host for the virus, that animal needs to have contact with bats, allow bat coronavirus to proliferate in them, and, eventually, the animal needs to have the capacity to transmit viruses to human beings who may or may not have contact with the Huanan seafood market. Therefore, there have been serious questions on whether this Wuhan coronavirus outbreak was due to a leak or mishandling of laboratory animals used in coronavirus studies. This is a reasonable public inquiry regarding the source of the outbreak and it warrants a transparent investigation from the Chinese authorities and foreign disease control and laboratory operation experts. This is not just about the accountability of medical ethics or laboratory safety operations, it is directly related to the current endeavors to contain the virus outbreak. While the animal host of 2019-nCoV is yet to be identified, the data and information from possible animal hosts and potential zoonic infection is imperative for prevention and controlling disease on an international scale. The Huanan seafood market has a high potential of harboring the animal host. Animal data and profiling results from the Huanan seafood market need to be disclosed immediately by Chinese authorities even if they are negative results. It is imperative for U.S. CDC and WHO officers to demand that Chinese authorities release the information about animal testing data. If Chinese authorities refuse to disclose testing data for animal samples, it could imply an intentional cover-up of the true origin of the 2019-nCoV outbreak.
https://spark.adobe.com/page/mKbM29QGon7kZ/ 来源:ZeroHedge,February 9, 2020 作者:XIANG ZHANG via The Epoch Times 新闻翻译:TCC, ignoreme, Roberts 评论:TCC 在短短两个多月的时间,武汉冠状病毒已经引起了中国三、四十个城市封城,世卫组织宣布为全球紧急事件,多国取消与中共航班等等,其影响不仅在公共卫生上,也对全球经济正带来未知的后果。 以流行病学及病毒学来说,病毒的起源以及可能宿主的掌握,对于流行病的控制是非常关键的。例如,如果老鼠是该病毒的中间宿主,那么在受感染的老鼠仍在自由活动的情况下,关闭城市以限制人们的行动是徒劳无功的。 疫情爆发已经两个月了,这个全球关注的起源问题,迄今扑朔迷离,众说纷纭。在这篇报导中,引用了多篇的学术论文与报告,从分子测序到蛋白分析比对,来找寻这病毒的源头。 蝙蝠与华南海鲜市场 病毒要建立人畜相传的传播并不是一件容易的事。基于演化史分析的报导,虽然此病毒基因序列与蝙蝠的冠状病毒非常相似,但一般冠状病毒很少会以高传播率从动物传播到人类的。并且蝙蝠冠状病毒直接跳传到人类的机会更渺小,尤其是武汉的大部分蝙蝠正在冬眠,且华南海鲜市场不卖蝙蝠。根据研究SARS(果子狸))与MERS(骆驼)的中间宿主所得的经验, 科学家又猜想是否这新病毒也有中间宿主?在一月一日华南海鲜市场关闭后,疑云重重,中共一直未提出动物采样的结果来进行分析讨论。关闭后,华南海鲜市场被打扫干净,’犯罪现场’消失了,如何在没有证据的情况下解决一个案件呢? 武汉病毒研究所与SARS样冠状病毒的研究 武汉新型冠状病毒在结构上带有非常重要的触突蛋白(S蛋白),据研究其结构与人类的血管紧张素转化酶2(ACE2)完全相同,可与人的细胞表面的ACE2受体结合,进而感染人类细胞。武汉病毒学研究所早在2013年《自然》杂志上发表的一篇文章,题为“使用ACE2受体的蝙蝠SARS样冠状病毒的分离和鉴定“。更巧的是,该研究室主要领导人石正丽是研究SARS样冠状病毒“功能获得”生物工程方面的权威。天然蝙蝠冠状病毒无法利用人类ACE2受体来感染人类。但是,如果在S蛋白序列某些位置插入一些氨基酸时,便可以利用人类ACE2受体,而造成感染。这在其2015表的文章,利用人工引发突变的新型冠状病毒可导致在哺乳动物模型中产生更致病,且更致命的冠状病毒株,可见一般。这对于人类的健康毫无帮助却有巨害, 因此被美国政府强制暂停这方面研究。但是否该研究所继续研究就不得而知了。 武汉新型冠状病毒动物宿主在华南海鲜市场的可能性 《柳叶刀》最新发表的一篇文章报道说:“冠状病毒在每个复制周期中都产生的突变的情况下,其平均进化速率约为每个位点每年10⁴个核苷酸取代。”也就是说,这种病毒每个复制周期都有突变,不同的蝙蝠作为宿主极不可能具有同样序列的病毒。如果还需要另一只动物作为中间宿主,这样病毒相同的机会甚至更小。令人惊讶的是,此篇描述了来自不同患者的病毒序列几乎完全相同。这在自然界中是不太可能发生的。 因此,对于武汉冠状病毒的爆发是否是由于冠状病毒的泄露或者对研究的实验动物处理不当,引起了严重的疑问。如果中共当局拒绝透露动物样本的检测数据,则可能意味着有意掩盖了2019-nCoV爆发的真实起源。 武汉冠状病毒的神秘起源中国继续拒绝发布动物抽样检测的数据 2 020年2月6日,中国湖北省武汉市的「火眼」实验室的一名实验室技术人员正在研究从病人样本中检测新冠状病毒。 分析 自从武汉冠状病毒爆发以来已经两个月了,它的传播在中国没有丝毫放缓的迹象。中共当局已经封锁了超过35个中国城市,以便隔离已确认和可疑的病例。随着该病毒显示出在中国以及国际上进一步传播的迹象,数百万人的生命处于危险之中。 中共官方调查对新型冠状病毒起源上存在很大落差。为了遏制这种病毒,首先需要了解这种被认为起源于动物的病毒是如何从动物感染到人的。为此,中共当局需要公布其动物测试数据和样本。从疫情中心点收集的动物样品的测试结果,可对哪些动物可以充当新冠状病毒的中间宿主,提供重要的见解。 这对于控制流行病至关重要。例如,如果老鼠是该病毒的中间宿主,那么在受感染的老鼠仍在自由移动的情况下,关闭城市以限制人们的行动是徒劳无功的。动物样本的结果也可以指导决策,以减少再次爆发的风险。 病毒的动物起源假说 基于演化史分析的科学报导,已经研究了新型冠状病毒的序列,并将其与其他冠状病毒序列进行了对比,发现它可能起源于蝙蝠。武汉病毒研究所的研究人员发现,在患者体内发现的病毒中的基因组,其中96%与现有蝙蝠冠状病毒的基因组相同。但是也有其他理论。例如,一项中国研究表明,蛇是向人类传播的来源。但是,许多科学家认为,爬行动物是一种不太可能的来源,而像大鼠,猪和一些鸟类等哺乳动物,一直是冠状病毒的主要宿主。 考虑到这一点,病毒基因组序列的演化史分析研究上,需要得到动物研究的支持,以确认感染的起源,并确定是否存在中间宿主。 病毒要建立人畜相传的传播并不是一件容易的事,并且冠状病毒很少会以高传播率从动物传播到人类的。而冠状病毒直接从蝙蝠向人类跳传的机会甚至更少。为了感染新宿主,病毒表面蛋白和/或病毒包膜和结构基因需要发生突变,所以突变的病毒可以结合并进入新物种的细胞,并有效地在新宿主中,完成复制周期。 一些科学家认为,冠状病毒可以直接跳传向人类,而不会变异或穿过中间物种。但是,以前的冠状病毒爆发上,显然需要中间宿主来建立人畜相传的传播方式。许多研究表明,在2003年SARS爆发期间,蝙蝠冠状病毒从其天然寄主蝙蝠跃传到果子狸,然后又跃传到人类,在MERS爆发时,它从蝙蝠跃传到骆驼,然后跃传到人类。因此,果子狸和骆驼被视为人畜相传的中间宿主。 由于蝙蝠在疫情爆发时并未在武汉的华南市场(感染的中心)出售,因此这表明存在着另一种中间动物宿主,该宿主可能已将病毒转移给人类。 最令人困惑的是,没有关于在武汉的任何疫情中心,尤其是在华南海鲜市场上,采集动物样本进行检测的报导。这是为了能鉴定哪些动物可能是这种新型武汉冠状病毒的宿主或中间宿主。 中国科学家最近在《柳叶刀》(Lancet)杂志上发表了一份报告,该报告指出:「大部分最早的病例包括报导曾接触过华南海鲜批发市场的病例」,这些患者可能是通过人畜共传或环境暴露的感染途径。在另一份《柳叶刀》杂志的报告,中国疾病预防控制中心的科学家声称, 「根据当前数据,造成武汉爆发的2019-nCoV似乎也可能是来自最初宿主的蝙蝠,并且有可能通过在华南海鲜市场上出售而目前未知的野生动物传播给人类的。」 但是,到目前为止,尚未公布任何关于关闭的华南海鲜市场的野生动物数量和种类的信息。也没有在2020年1月1日市场关闭时,任何关于如何管理或处置这些动物的信息。更没有发布有关多少动物样本通过病毒核酸检测方法检测SARS-CoV或武汉冠状病毒的信息。 中共官方新闻机构新华社1月26日报导,在华南海鲜市场采集的585个环境样本中,有33个样本的新冠状病毒核酸检测呈阳性,这表明该病毒源自野生动物或在在市场出售的种群。但是,这些样本来自环境,而不是直接从动物的。 如果在关闭造成疫情爆发的华南海鲜市场时或关闭之前,没有收集和测试动物样本,这将是武汉市公共卫生委员会和中共疾病预防控制中心的大失败。这将类似于对食源性疾病爆发进行调查,却没取得与疾病爆发相关的饭店食品样本,而是只对餐桌表面的拭子进行测试。 华南海鲜市场关闭的背景 2019-nCoV在中国引起了快速感染,并传播到中国以外的其他国家,从而引发了全球卫生危机。 众所周知,华南海鲜市场是收集和分发活的和死的野生动物的主要渠道。其中包括在海鲜市场东部出售的活狼,刺猬,鹿,鸟,蛇,山羊,野兔和野猪。 武汉市医疗卫生委员会于2019年12月31日宣布了多例与华南海鲜市场有关的肺炎病例。武汉市政府于1月1日关闭了该海鲜市场。 中国医学记者于2019年12月31日,即1月1日该市场关闭的前一天晚上访问了该市场,发现那里的卫生状况极差,野生动物的尸体和器官随意乱丢。这表明在强制关闭后市场上仍存在相对大量的野生动物。 没有公布关于海鲜市场野生动物的信息 然而,在1月1日市场关闭时,对于存在动物的数量和种类,多少动物被测试了冠状病毒,以及在1月1日市场关闭后如何管理或处置这些动物的信息,都未公布。中共媒体,第一财经,询问了在市场上出售的野生动物的下落,并确定武汉市政府完全没有披露。 香港大学新兴传染病国家重点实验室现任(中国事务)主任关毅博士,于1月21日访问武汉,目的是确定动物来源。他在接受媒体采访时提到,当地人拒绝与他合作。他指出,由于现在市场已经关闭,很难调查该病毒的来源。他说:「关闭后,湖南海鲜市场被打扫干净,’犯罪现场’消失了,如何在没有证据的情况下解决一个案件呢?」 中共疾病预防控制中心主任高福说:「很明显,感染源是野生动物,但由于海鲜市场关闭,我们不知道是哪种物种。」 没有确认病毒来源或动物中间宿主带来的巨大风险 美国疾病控制中心(CDC)表示:「对于新型冠状病毒如何传播我们知之甚少」目前了解到的是新型冠状病毒的感染是通过呼吸道飞沫或者是人与人之间的接触。 香港大学的Guan Yi 和Kwok-yung Yuen等在2003年从中国活体动物交易市场(售卖)的果子狸身上识别出严重的急性呼吸系统冠状病毒(SARS-CoV)。他们的研究致使广东禁止贩卖果子狸并关闭了野生动物交易市场。这有助于限制SARS疫情。 通常来说,如果一个动物被鉴定为疾病的宿主或者传播源,主管部门和CDC将会开始实施预防和控制措施,比如,宣传,对生病动物进行适当的检疫隔离,和处理病死动物尸体,同时监控潜在的人畜相传的传播途径。 啮齿类动物是已知的会在海鲜市场衍生。比如,随着日本一个大型鱼类市场的关闭,成千上万的啮齿类动物会被释放出来。 华南海鲜市场也被啮齿动物侵扰。如果啮齿动物被视为冠状病毒潜在宿主,则鼠类在隔离区外活动也存在风险。鉴于深圳患者的粪便中被检测出冠状病毒,并且蝙蝠类SARS病毒株从蝙蝠粪便中被分离出来,所以除了呼吸道飞沫传播,2019新型冠状病毒也可能通过粪-口传播,需要警告人们避免接触老鼠这样的动物。因此,如果啮齿动物确实是2019新型冠状病毒感染的源头或宿主,那么需要引起国际社会的注意。 同样的,如果海鲜市场的鸟类或其他类动物是新型冠状病毒的宿主,则动物的种类,数量,病毒类型,生物学反应和病毒潜在传播途径等相关信息需要被认定和报告给国际社会,以便采取相应的防范措施。 如果中共当局没有从海鲜市场贩卖的动物和鸟类身上收集鼻腔分泌物,粪便和血液样本,这会是无能和严重的渎职行为。对于人畜相传传播途径,这次爆发中的病毒变异趋势,和当前措施的漏洞,对动物样本检测会揭示出非常重要的信息。 除了华南海鲜市场,还有其他的爆发中心吗? 中共疾病预防控制中心确实发布了来自于海鲜市场环境样本的数据,并建议这里的野生动物(不确定物种)是病毒源头。 2020年1月29日,包括中共疾控中心的冯博士的一个小组在《新英格兰医学杂志》发布了一篇题为《新型冠状病毒感染肺炎在中国武汉的早期传播动力学》的报告。报告称「虽然最早的病例大部分与华南海鲜批发市场相关,并且这些病患可能是通过人畜或者接触到其环境被感染…大部分的最早期病例被报告接触过华南海鲜市场,但是,没接触过海鲜市场的病例数量在12月底成指数倍增长。」 2019新型冠状病毒来自于蝙蝠类SARS病毒(Bat-SL-CoV)的可能性 在《柳叶刀》杂志2020年1月29日发表的一篇文章,题为「2019新型冠状病毒的基因组特征和流行病学:病毒源头和受体结合的意义」称:「对2019新型冠状病毒完整的基因组进行的海量搜寻揭示了基因库中可用的相关病毒中最接近的是蝙蝠类SARS冠状病毒:bat-SL-CoV-ZC45(序列相同性87.99%,查询覆盖率99%)以及另外一个来自蝙蝠的SARS样β冠状病毒:bat-SL-CoV-ZXC21(登记号:MG772934;23 相同性87.23%)。」 「值得注意的是,2019新型冠状病毒株与SARS和MERS冠状病毒的基因相似度不高(分别大约是79%和50%)。」 这个信息可能解释了2019新型冠状病毒与来源于蝙蝠的SARS 样β冠状病毒具有很近的生物学相关性,而且蝙蝠可能是此病毒的原始宿主。然而,作者没有明确指出蝙蝠是2019新型冠状病毒的唯一宿主。 该论文指出「蝙蝠具有重要性,但几个事实表明其他动物也可能是蝙蝠与人类之间的中间宿主。首先,此次爆发在2019年12月底被首次报告,武汉的大部分种类的蝙蝠正在冬眠。其次,华南海鲜市场并没有发现售卖蝙蝠,但是贩卖各种非水生动物包括哺乳动物。第三,2019新型冠状病毒与近亲bat-SL-CoVZC45和bat-SL-CoVZXC21 间的序列同一性小于90 %,这反应了它们之间有相对较长的分支,这两种病毒不是2019新冠状病毒的直接祖先。第四,对于SARS和MERS冠状病毒,蝙蝠是其自然携带者,其他动物是(果子狸是SARS-CoV35的,单峰骆驼是MERS-Cov的)中间宿主。因此,在现有数据基础上,蝙蝠非常可能是武汉爆发的2019新型冠状病毒的原始宿主,且有可能通过华南海鲜市场售卖的其他现在还未知的野生动物传给人类的。」 他们提到,武汉的大部分蝙蝠正在冬眠,且华南海鲜市场也没有售卖蝙蝠。因此通过与蝙蝠的直接接触来传播病毒给人或其他动物的可能性是极小的。 武汉病毒研究所对蝙蝠类SARS冠状病毒的研究 石正丽和武汉病毒学研究所的其他几位研究人员于2013年在《自然》杂志上发表了一篇文章,题为「使用ACE2受体的蝙蝠SARS样冠状病毒的分离和鉴定」。 在该研究中,他们的团队从蝙蝠的肛门拭子或粪便样本中收集了2株来自蝙蝠SARS样冠状病毒的序列,分别称为RsSHC014和Rs3367。他们与人类SARS-CoV Tor2菌株核苷酸序列有95%的相似性。 2015年12月30日发表在《病毒学杂志》上的一项研究中,名为「与严重急性呼吸系统综合症冠状病毒直接源细胞密切相关的新型蝙蝠冠状病毒的分离和鉴定」的研究发现了一种新的病毒,名为SL-CoV-WIV1,其基因与Rs3367几乎相同,具有99.9%的基因组序列相似性。研究人员确定,WIV1可以使用人类ACE2作为进入受体,并有可能感染人类细胞。随后,同一研究小组于2015年在实验室中分离出了另一种可以使用ACE2并感染人类细胞系的蝙蝠病毒。 此外,石博士的小组于2018年进行了另一项研究,以解决一些蝙蝠病毒是否可以通过使用人ACE2感染人类而无需中间宿主的问题。但是到迄今他们的研究,「尚未有类似SARS的冠状病毒可以从蝙蝠直接传给人类的报导」。 他们从居住在带有病毒的蝙蝠洞附近的218名居民中收集了血清,那些洞穴是石博士小组收集病毒样本的地方。然后进行ELISA分析,以检测抗蝙蝠SARS冠状病毒的抗体,如果抗体存在,则表示事先暴露于蝙蝠冠状病毒。他们发现,在218名受试者中,只有6名(2.7%)显示出血清阳性,这表明可能感染了蝙蝠SARS-CoV或相关病毒。在过去的12个月中,这6名阳性患者没有临床症状。他们从距云南1000公里处的武汉市的随机献血者那里收集了240个样本作为对照组,武汉市的血液样本对SARS样冠状病毒全没有显示出阳性。 该数据表明,蝙蝠病毒感染人类的机会非常低,甚至在生活于蝙蝠洞附近的人群中也只小于2.9%的机会,并且(这些感染者)没有明显的症状。截至2018年,武汉市并无蝙蝠感染人类的报告。 [ZH:2020年2月,她的研究小組在《自然》雜誌上發表了一篇論文,表明吉利德科學公司擁有的實驗藥物瑞得西韋對體外抑制病毒產生積極作用,並代表武漢病毒研究所在中國申請了該藥物的專利。] 武汉病毒研究所在蝙蝠SARS样冠状病毒「功能获得」生物工程方面亮丽的成绩 武汉市病毒研究所的石正丽研究小组成功地从蝙蝠中分离出两个蝙蝠SARS样冠状病毒的传染性克隆:SL-CoV-WIV1和WIV16。在进一步的研究中,他们发现这些SL-CoV 突触蛋白(S蛋白)「无法使用三种ACE2分子中的任何一种作为其受体。其次,SL-CoV无法进入表达蝙蝠ACE2分子的细胞中。第三,覆盖先前定义的受体结合域的嵌合体S蛋白通过人类ACE2分子获得了进入细胞的能力,尽管对不同构建体的效率不同。第四,发现最小的插入区(氨基酸310至518)足以将SL-CoV S蛋白从非ACE2分子结合转化与人类ACE2分子结合。」 因此,石的小组在2008年2月发表在《病毒学杂志》上的一项研究中发现,天然蝙蝠冠状病毒无法利用人类ACE2受体来感染人类。但是,当在蝙蝠CoV S蛋白序列310到518的位置插入一些氨基酸时,嵌合体蝙蝠CoV可以使用人类ACE2受体。 同时,由李博士领导的另一个研究小组于2013年发表了他们的发现,冠状病毒突触蛋白上的5个氨基酸位点对SARS病毒与人类ACE2的结合至关重要(位置分别为Y442,L472 ,N479,D480,T487) 。这五个点就在石小组在上面指出的重要区域内。 后来,李和石于2015年9月在《病毒学杂志》上联合发表了项「功能获得」的研究。该研究于2015年针对MERS病毒和一种蝙蝠病毒(HKU4株)进行了研究。他们在HKU4突触蛋白中引入了2个单突变,发现新的突变S蛋白可以使HKU4进入人体细胞。如果他们在MERS S蛋白突变2个位点,则产生的MERS伪病毒(实验病毒)将无法再进入人类细胞。 此外,石博士的小组加入了一个国际小组,共同用云南收获的蝙蝠病毒SHC014产生嵌合病毒。因为他们知道SHC014不太可能与人类ACE2结合,所以他们「在具有复制能力的小鼠适应SARS-CoV骨干的背景下合成了SHC014突触蛋白」。因此,这是一种具有SARS-CoV小鼠适应性骨干(MA15)但带有SHC014突触的实验室工程病毒。 令他们惊讶的是,嵌合病毒(SHC014-MA15)可以使用SHC014突触结合人类ACE2受体并进入人的细胞。 SHC014-MA15也可引起小鼠疾病并引起死亡。现有的SARS疫苗不能保护动物免受SHC014-MA15感染。因此,这些嵌合病毒研究可导致在哺乳动物模型中产生更致病,且更致命的冠状病毒株。 由于美国政府强制暂停「功能获得」(GOF)研究,因此该国际研究当时并未进一步进行。但是,没有证据显示石博士在中国的小组停止了在CoV中引入GOF突变的进一步研究。很明显,石的研究小组已经掌握了逆向工程技术,该技术足以在当前的SARS冠状病毒或SARS 样冠状病毒中引入突变以产生突变型的传染性冠状病毒。 [ ZH:2020年2月,《南華早報》報道說,石正麗進行了長達十年的工作,為建立全球最大的蝙蝠相關病毒數據庫之一,並為科學界提供了瞭解該病毒的「開端」。 《南華早報》還報道說,石是中國社交媒體上人身攻擊的焦點,他們聲稱武漢病毒研究所是該病毒的來源,這導致石聲稱:「我用我的生命發誓,[該病毒]与实验室无关。 」她并且在《南华早报》要求对这些攻击发表评论时,石答道:「我的时间必须花在更重要的事情上。」 《财新》报道说,石进一步发表了公开声明,反对「有关新病毒来源的阴谋论」,并引用她的话说: 「 新的2019冠状病毒是对人类不文明的生活习惯的一种惩罚。我,石正丽,用我的生命担保与我们的实验室无关」] 有趣的是,石的研究小组于2020年1月23日在bioRxiv上发表了文章,他们在云南发现的一种新的蝙蝠冠状病毒,名为BatCov RaTG13,与2019-nCoV有96.2%的整体基因组序列相同性。但是,这种病毒从未在他们的研究中提及或发表过。 在一系列他们提供的补充材料和方法信息中,他们收集的2019-nCoV与RATG13病毒之间共享3个序列,却与其他SARS或蝙蝠SARS 样病毒系列中的任何一个也不共享。这3个序列位于突触蛋白的N末端附近,分别是GTNGTKR,NNKSWM,RSYLTPGD。 2019-nCoV动物宿主在华南海鲜市场的可能性 《柳叶刀》最新发表的一篇题为「 2019年新型冠状病毒的基因组特征和流行病学:对病毒起源和受体结合的影响」的文章报道说:「作为典型的RNA病毒,冠状病毒在每个复制周期中都产生的突变的情况下,其平均进化速率约为每个位点每年10⁴个核苷酸取代。因此,令人惊讶的是,此处描述的来自不同患者的2019-nCoV序列几乎相同,具有超过99.9%的序列相同性。这一发现表明,2019-nCoV在很短的时间内具有同一个来源,并且被相对迅速地发现。」 由于每个周期都有突变,不同的蝙蝠作为宿主极不可能具有同样序列的病毒。如果仅仅蝙蝠不足以传播病毒,则需要另一只动物作为中间宿主,这样病毒相同的机会甚至更小。由于海鲜市场并非爆发的唯一来源,因此可以合理地假设,如果另一只动物是该病毒的中间宿主,则该动物需要与蝙蝠接触,允许蝙蝠冠状病毒在其中繁殖,并在最后,该动物需要具备将病毒传播给与华南海鲜市场接触或没有接触的人的能力。 因此,对于武汉冠状病毒的爆发是否是由于冠状病毒的泄露或者对研究的实验动物处理不当,引起了严重的疑问。对疫情来源的合理公开查询,值得中共当局、外国疾病控制和实验室操作的专家进行透明调查。这不仅与医学伦理或实验室安全操作的责任制有关,还与遏制病毒爆发的当前努力直接相关。 尽管尚未确定2019-nCoV的动物宿主,但可能的动物宿主和潜在的人畜共传的数据和信息对于在国际范围内预防和控制疾病至关重要。 华南海鲜市场蕴藏着巨大的动物宿主群。华南海鲜市场的动物数据和分析结果需要由中共当局立即披露,即使结果是阴性的。美国疾病预防控制中心和世界卫生组织官员必须要求中共当局发布有关动物试验数据的信息。 如果中国当局拒绝透露动物样本的检测数据,则可能意味着有意掩盖了2019-nCoV爆发的真实起源。 |
|
|
|
|
 | |
|
 |
| 实用资讯 | |
|
|
|
|
| 一周点击热帖 | 更多>> |
|
|
|
| 一周回复热帖 |
|
|
|
|
| 历史上的今天:回复热帖 |
| 2019: | 国家计生委依据中国宪法拒绝退出历史舞 | |
| 2019: | 文庙错了英国记者被僧格林沁剁成了60块 | |
| 2018: | BBC| 阴谋论、假新闻、网络谣言为何能 | |
| 2018: | Safari——精彩纷呈的动物世界 | |
| 2017: | Melanie 的确内向拘谨,没有亲和感。 | |
| 2017: | 北加很快被fucked up | |
| 2016: | 这两天网上热贴,上海女到江西男友家过 | |
| 2016: | 去准婆家之前,肯定对综合指标多次衡量 | |
| 2015: | 不择手段、杀人利己的生动解说 | |
| 2015: | 涯:印象 69(2)荒诞斗校长 | |